| code | description | presentation | sterile | case qty. | case weight (Kg) | case vol. (m3) | tube length | Ø exterior |
|---|---|---|---|---|---|---|---|---|
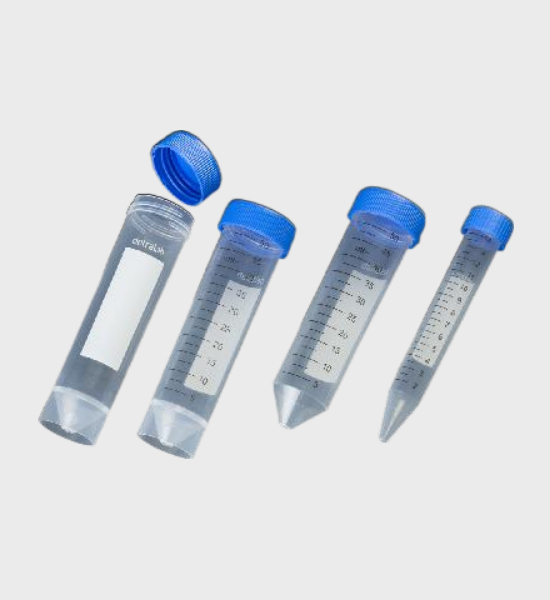
| 429940 | 15ml tube non skirted | 500 tubes in bulk | NO | 500 | 4,50 | 0,0281 | 121 mm | 16.5 mm |
| 429945 | 15ml tube non skirted | 20 bags of 25 units | NO | 500 | 4,50 | 0,0281 | 121 mm | 16.5 mm |
| 429942* | 15ml tube non skirted | 20 bags of 25 units | STERILE R | 500 | 4,50 | 0,0281 | 121 mm | 16.5 mm |
| 429930 | 50ml tube non skirted | 20 bags of 25 units | NO | 500 | 7,70 | 0,0844 | 116 mm | 29.2 mm |
| 429931* | 50ml tube non skirted | 20 bags of 25 units | STERILE R | 500 | 7,44 | 0,0820 | 116 mm | 29.2 mm |
| 429950 | 50ml tube skirted | 20 bags of 25 units | NO | 500 | 8,80 | 0,0844 | 117.2 mm | 29.2 mm |
| 429951* | 50ml tube skirted | 20 bags of 25 units | STERILE R | 500 | 8,38 | 0,0820 | 117.2 mm | 29.2 mm |
Regulation (EU) 2017/746. “In vitro” diagnostic medical devices (IVDR). DNAsa, RNAsa and pyrogen free.
*Directive 98/79/CE. “In Vitro” diagnostic medical devices (IVDD).









Reviews
There are no reviews yet.